One of the most well known qualities of the ocean is that it is salty. The two most common elements in sea water, after oxygen and hydrogen, are sodium and chloride. Sodium and chloride combine to form what we know as table salt.
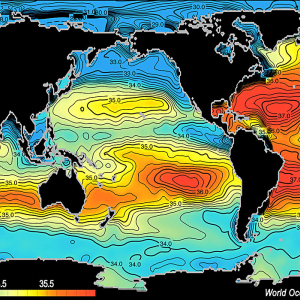
Sea water salinity is expressed as a ratio of salt (in grams) to liter of water, It is written parts per thousand (ppt). In sea water, there is typically close to 35 grams of dissolved salts in each liter (35ppt), but ranges between 33-37 grams per liter (33ppt - 37ppt).
But as in weather, where there are areas of high and low pressure, the ocean has areas of high and low salinity. Of the five ocean basins, the Atlantic Ocean is the saltiest. On average, there is a distinct decrease in salinity near the equator and at both poles, although for different reasons.
Near the equator, the tropics receive the most rain on a consistent basis. As a result, the fresh rain water falling into the ocean decreases the salinity of the surface water in that region. Rain decreases further from the equator, and with less rain and more sunshine, evaporation increases. Evaporation of water vapor from the ocean to the atmosphere leaves behind the salt, resulting in higher salinity. Toward the poles, fresh water from melting ice decreases the surface salinity once again.
The saltiest locations in the ocean are the regions where evaporation is highest or in large bodies of water where there is no outlet into the ocean. The saltiest ocean water is in the Red Sea and in the Persian Gulf region (around 40ppt) due to very high evaporation and little fresh water inflow.
Take it to the MAX! "A Funny Bath" - The Dead Sea
Learning Lesson: "A Funny Taste"
Floating sea ice is the result of a unique property of water. As the temperature decreases to 40°F (4°C), the molecules slow and contract, and the density increases, same as other substances. Below 40°F (4°C), however, the water molecules begin to bond to each other; as they do, they are held apart, and the water expands again, decreasing the density. At 32°F (0°C), all molecules are locked into a crystalline structure, resulting in a nine percent expansion in size. This expansion and corresponding decrease in density is the reason ice floats.
Take it to the MAX! Titanic Bergs
The amount of salt in sea water determines the temperature at which sea water freezes because adding salt to water lowers the freezing temperature. Water with a salinity of 17ppt freezes at about 30°F (-1°C) and 35ppt water freezes at about 28.5°F (-2°C). However, sea ice itself contains very little salt, about a tenth of the amount of salt that sea water has. This is because ice will not incorporate salt into its crystal structure. Newly formed sea ice can trap pockets of salty water, called brine, making the ice salty at first. Eventually, though, the brine gets pushed out of the ice’s crystal structure. Therefore, older sea ice is actually drinkable.
Learning Lesson: We all Scream for Ice Cream
The density of sea water, however, is influenced by both its temperature and salinity. Density increases as salinity increases and as temperature decreases. Because salt lowers the freezing point of water, sea water does not start forming the lattice structure that lowers its density until it is much colder than fresh water. Therefore, when sea ice does form and loses salt, the salt is concentrated in the water beneath it, and the salinity (and therefore the density) of the underlying water continues to increase well after an area is iced over.
Learning Lesson: Salt 'n Lighter
The "Average Salinity" map (right) shows the lowest salinity being in the polar regions. However, this image depicts surface salinity only. Polar surface salinity is lower than in the tropical regions due to ice melting each summer. However, winter ice formation increases the salinity below the ocean surface, causing the water below the ice to sink. That sinking motion governs the flow of the ocean's deep-water currents.
Learning Lesson: Diet Light